LUXEMBOURG CITY, Luxembourg — Backed by the eradicateMalaria initiative, German company BioNTech announces a malaria project consisting of the development of an mRNA-based vaccine against the disease and the evaluation of mRNA vaccine production in Africa; The initiative can count on substantial financing by Team Europe, including for the late-stage trials of BioNTech’s project; eradicateMalaria is run by the kENUP Foundation and benefits from the convening power of the World Health Organization and the Africa Centre for Disease Control and Prevention.
Backed by the eradicateMalaria initiative, German biotech company BioNTech SE (https://bit.ly/3iQ6Zdq) (“BioNTech”) announced a project to develop a malaria vaccine candidate based on its proprietary mRNA technology today. The project also aims to expand vaccine production capacity across Africa. BioNTech co-developed the first mRNA-based COVID-19 vaccine with its partner Pfizer. The company has benefited from two European Investment Bank (EIB) loans under the Investment Plan for Europe (https://bit.ly/3zC2UQJ) for its cancer and COVID-19 research.
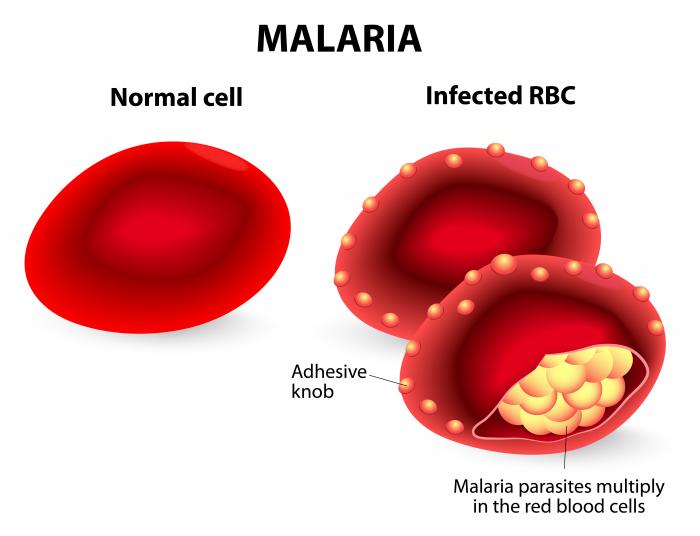
The scientific and entrepreneurial progress made during the pandemic raises hopes that a highly efficacious vaccine could soon help to eradicate malaria. This goal was out of reach so far, despite the enormous financial and public health efforts of the global community. BioNTech is the first major vaccine developer in over 30 years to commit to working towards eradicating malaria. The company follows a two-pronged approach. 1) It aims at developing a first-generation mRNA (https://bit.ly/3ycwDzC) vaccine, using a known antigen, the CSP protein. The clinical trial for this first-generation vaccine candidate is expected to start at the end of 2022. 2) It will be running a dedicated antigen discovery process to potentially identify new antigens, which may pave the way for a second-generation vaccine with higher efficacy. In addition, the company has pledged to manufacture the potential vaccine in African facilities – either with licensed production partners or on its own.
BioNTech plans to use revenues from the COVID-19 vaccine to develop its malaria vaccine candidates and bring them to the first phase of clinical trials. The EIB and the European Commission pledge to support companies that aim to eradicate malaria via the joint InnovFin Infectious Diseases Finance Facility (https://bit.ly/3eS6ZIH), backed by Horizon 2020. EIB investment will cover projects that enter late-stage clinical development, which primarily aims at demonstrating the efficacy, safety and cost-effectiveness of a medical product.
“Malaria is a tricky disease to vaccinate against – this is why it takes a lot of courage and dedication to embark on the endeavour BioNTech just committed to,” said Werner Hoyer (https://bit.ly/3eYD3up), President of the EIB. “Finding an efficient vaccine is the only way to eradicate one of the biggest causes of death in children in less developed countries. mRNA technology has shown itself to be a game changer to end the pandemic, and the EIB confirmed its support for this innovative approach with two loans to BioNTech, one in 2019 for developing cancer treatments and the other in 2020 for research on the COVID-19 vaccine. If mRNA can revolutionise malaria vaccine development as well, the EU bank would be proud to support this mission.”
Ursula von der Leyen (https://bit.ly/3iRiW2i), President of the European Commission: “We are witnessing the start of a revolution in medical science – the revolution of messenger RNA technology, pioneered by BioNTech and others. Thanks to this, billions of doses of a COVID-19 vaccine are being produced for Europe and the world. And mRNA technology can be a game changer in the fight against other diseases too – including malaria. Eradicating malaria is a realistic goal and now we know that it might be achieved already in this generation. The European Commission is supporting the global effort to develop mRNA vaccines against malaria. This initiative is also a part of the broader engagement by the EU for health in Africa and the Developing World. If we succeed, we will not only be better equipped for the next pandemic. We will also invest in an African continent that is finally free from malaria.”
Mariya Gabriel (https://bit.ly/3BG6hbg), Commissioner for Innovation, Research, Culture, Education and Youth: “For decades, the European Union’s research and innovation programmes along with its financial instruments have provided the framework and financing to contribute to the global research agenda for malaria, and we are committed to continuing these efforts. Solving the global threats of malaria requires breakthrough discoveries, the efforts of our brightest minds, and also the joint actions of policymakers and investors. Today, I am very pleased to embark on a new venture with our global partners with the aim of bringing an mRNA-based vaccine against malaria from the idea to the patient to address and eradicate the disease once and for all.”
Jutta Urpilainen (https://bit.ly/3i3wubO), Commissioner for international partnerships: “I warmly welcome the ground-breaking announcement of BioNTech, that aims to use the mRNA technology in the fight against Malaria, a major disease affecting the African continent. Our Team Europe initiative on enhancing vaccine manufacturing and access to medicines and health technologies in Africa will support this important project.”
Uğur Şahin (https://bit.ly/3i4Ahpp), CEO and co-founder of BioNTech, said: “We’re committed to reducing the suffering of people worldwide, so we feel we have a duty to utilise our technology to develop and manufacture an mRNA-based vaccine that addresses this life-threatening disease. We want to develop sustainable solutions for and together with the people of Africa. Setting up infrastructure could help to address various diseases using this disruptive technology. Building on our mRNA technology and the expertise gained during the pandemic, our efforts will include substantial investments in vaccine development as well as transferring manufacturing expertise to sites on the African continent.”
In parallel to developing a malaria vaccine, BioNTech will evaluate how to establish sustainable mRNA manufacturing capabilities on the African continent. The company plans to co-locate its potential African facilities with the technology transfer hubs under development by the World Health Organization (WHO). The hubs will strengthen low- and middle-income countries’ capacity to manufacture COVID-19 vaccines and increase global vaccine production. BioNTech’s commitment to vaccine production on the African continent supports Team Europe’s Sustainable Healthcare Industry for Resilience in Africa (SHIRA) initiative (https://bit.ly/3rECevR), as the company’s projected mRNA vaccine manufacturing facilities could also produce the existing vaccine against COVID-19 or an envisioned tuberculosis vaccine – pending successful development of candidates and regulatory approval.
The fight against malaria has been one of the European Union’s priorities in the health and development sectors for some years now. In 2019, the EIB and the European Commission supported the EU Malaria Fund, a public-private partnership between the European Union, international organisations, corporations, and civil society, initiated by the kENUP Foundation. With the advancement of the BioNTech malaria vaccine candidate on a proven technology platform, the EU Malaria Fund accomplished its mission earlier than expected. Therefore, on June 30, its investment period came to an end. The fund has successfully initiated more than two dozen novel scientific approaches to fighting malaria and financed several innovative companies.